Abstract
Background: This review will evaluate the efficacy of medical marijuana as a therapy for women with postpartum depression (PPD). PPD will be defined as a major depressive disorder that arises up to 30 days after childbirth, with the diagnosis made by a depression questionnaire. PPD is caused by a hormonal imbalance in the female hypothalamic-pituitary axis (HPA) after childbirth. Current treatments include pharmacologic and nonpharmacologic methods, which have demonstrated symptom improvement. Our team is interested in the use of medical marijuana as a potential pharmacologic alternative for PPD, due to current research that demonstrates the involvement of the endocannabinoid system in psychiatric disorders.
Objectives: The purpose of this study is to review the efficacy of medical marijuana as a treatment for PPD.
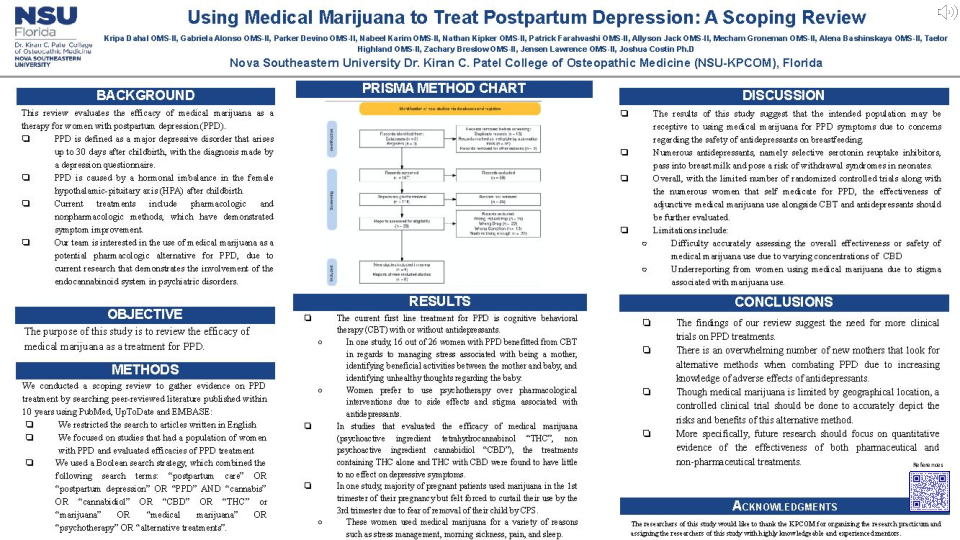
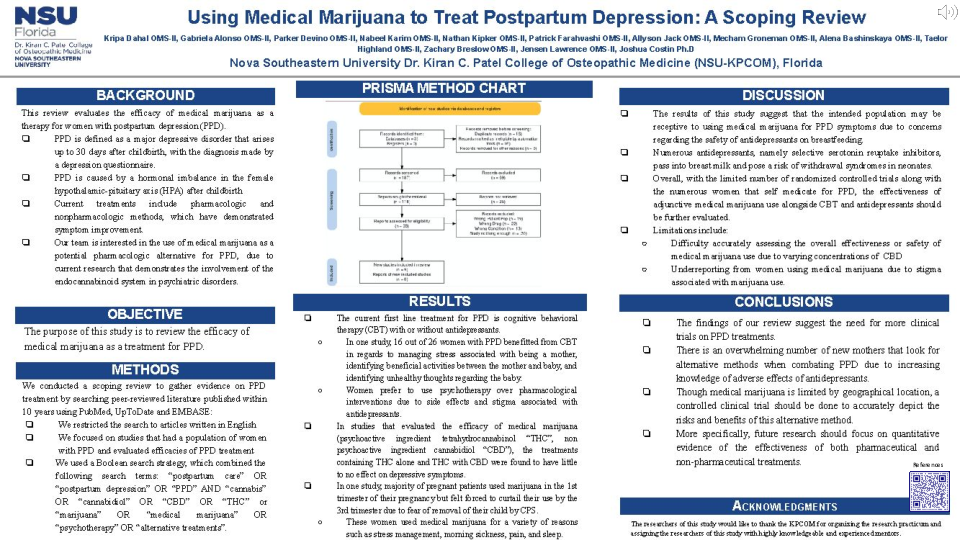
Methods: We conducted a scoping review to gather evidence on PPD treatment. We searched peer-reviewed literature published within 10 years using PubMed, UpToDate and EMBASE. We restricted the search to articles written in English. We focused on studies that had a population of women with PPD and evaluated efficacies of PPD treatment. We used a Boolean search strategy, which combined the following search terms: “postpartum care” OR “postpartum depression” OR “PPD” AND “cannabis” OR “cannabidiol” OR “CBD” OR “THC” or “marijuana” OR “medical marijuana” OR “psychotherapy” OR “alternative treatments”.
Results: After evaluating the evidence collected, the current first line treatment for PPD is cognitive behavioral therapy (CBT) with or without antidepressants and in the studies evaluated, the control groups received CBT. In studies that evaluated the efficacy of medical marijuana (active ingredient tetrahydrocannabinol “THC”), the treatments containing THC alone were found to have little to no effect on depressive symptoms. Overall, with a very limited amount of randomized controlled trials, medical marijuana was found to be an effective secondary therapy when used in conjunction with CBT and antidepressants.
Conclusion: The findings of our review suggest the need for more clinical trials on PPD treatments. There is an overwhelming amount of new mothers that look for alternative methods when combating PPD due to increasing knowledge of adverse effects of antidepressants. Though medical marijuana is limited by geographical location, a controlled clinical trial should be done to accurately depict the risks and benefits of this alternative method. More specifically, future research should focus on quantitative evidence of the effectiveness of both pharmaceutical and non-pharmaceutical treatments.