Abstract
Alexander Louie1, George Rodrigues2, Cornelis Haasbeek1, Andrew Warner2, Frank Lagerwaard1, David Palma2, Ben Slotman1, Suresh Senan1
1VU University Medical Center, Amsterdam, Netherlands
2London Regional Cancer Program, London, ON
Purpose: Stereotactic Ablative Radiotherapy (SABR) is now a guideline-specified curative treatment modality for patients with early stage non-small cell lung cancer (NSCLC). This analysis was undertaken to develop a prognostic model for overall survival (OS) in these patients using recursive partitioning analysis (RPA).
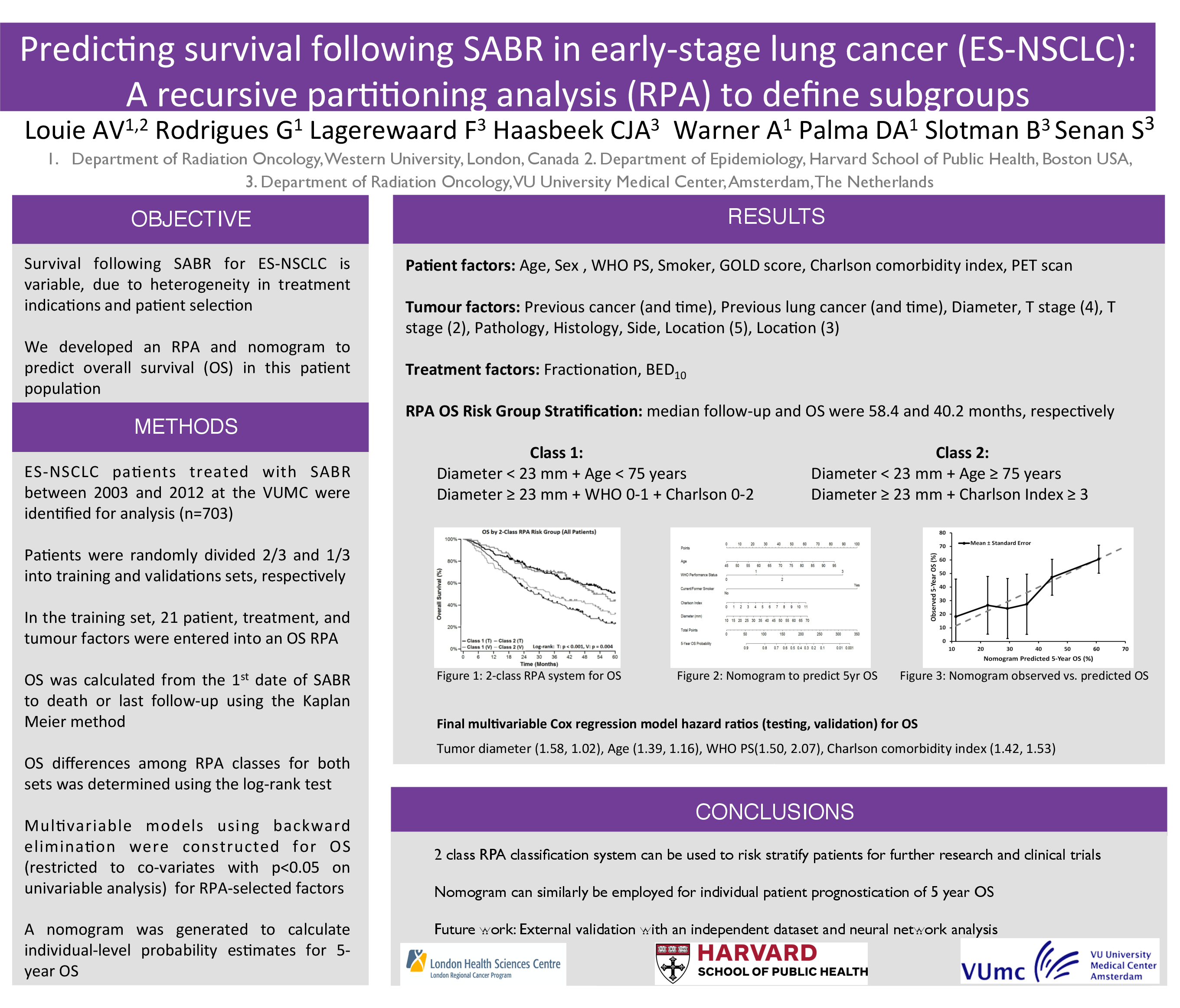
Materials and Methods: Our institution maintains a database on lung tumour patients treated with both SABR and hypofractionated radiotherapy. Details on baseline characteristics, treatment, and follow-up information are prospectively entered. SABR was delivered using a risk-adapted scheme of 54 Gy in 3 fractions, 55 Gy in 5 fractions, 60 Gy in 8 fractions, and the hypofractionated scheme used with stereotactic on-line setup was 60 Gy in 12 fractions, all based on tumour size and location. The following categories of patients were excluded: previous lung cancer, other synchronous malignancies, multiple lung tumours, and those without 18-FDG PET staging. 676 Stage I NSCLC patients treated between 2003 and 2012 remained eligible for analysis, consisting of both medically inoperable and potentially operable patients, with the latter defined by criteria described previously [Lagerwaard 2012]. Patients were randomly dividing into a training set (n=451, 67%) and a validation set (n=225, 33%). In the training set, 22 unique parameters consisting of various patient, treatment, and tumour factors were entered into a model where recursive partitioning was used to prognosticate for OS. After selection of a clinically appropriate model, classes developed in the training set were applied to stratify patients from the validation set. The log-rank test was used to determine differences in OS among the RPA classes for the validation and training sets.
Results: At a median follow up of 25.5 months (range 0.9-113.9), the median OS for the entire cohort was 51.3 months. RPA identified six risk classes: class I - potentially operable and PTV < 45 cc; class II - potentially operable and PTV > 45 cc; class III - medically inoperable and diameter < 2.4cm and age <76; class IV: medically inoperable and diameter <2.4cm and age >76; class V: medically inoperable, diameter >2.4 and BED10 >100 Gy; and class VI medically inoperable, diameter >2.4 and BED10 <100 Gy. In the training set, median OS was not reached for class I, and for classes II-VI were 58.7, 44.0, 25.8, 20.5, and 10.0 months, respectively. The RPA model could significantly discriminate between risk classes in both the training and validation sets (p<0.0001). Overall survival in the validation set correlated closely with survival in the training set.
Conclusions: This validated model demonstrates that operability, tumour diameter, PTV size, BED10 and age form the basis of a new risk stratification for OS in Stage I NSCLC patients treated with SABR that may help define subgroups for future clinical trials.