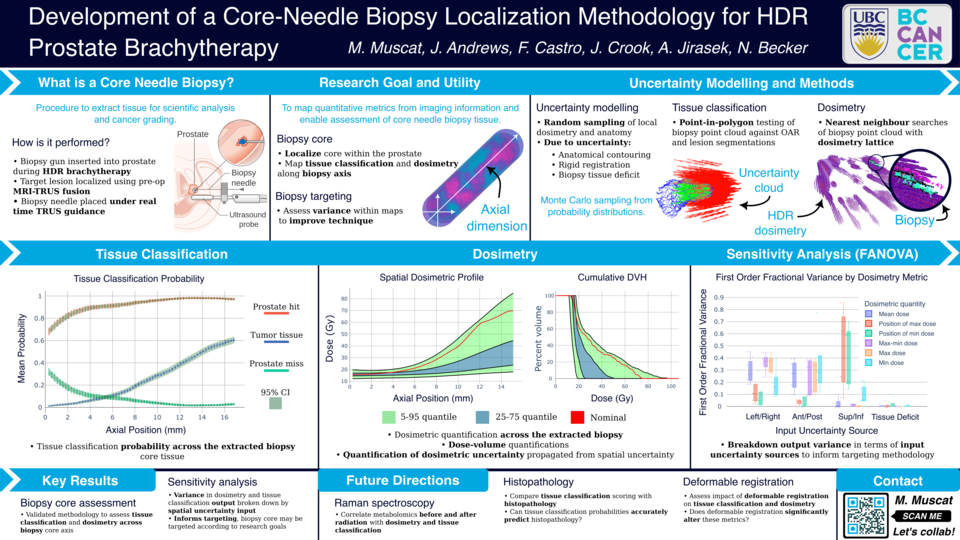
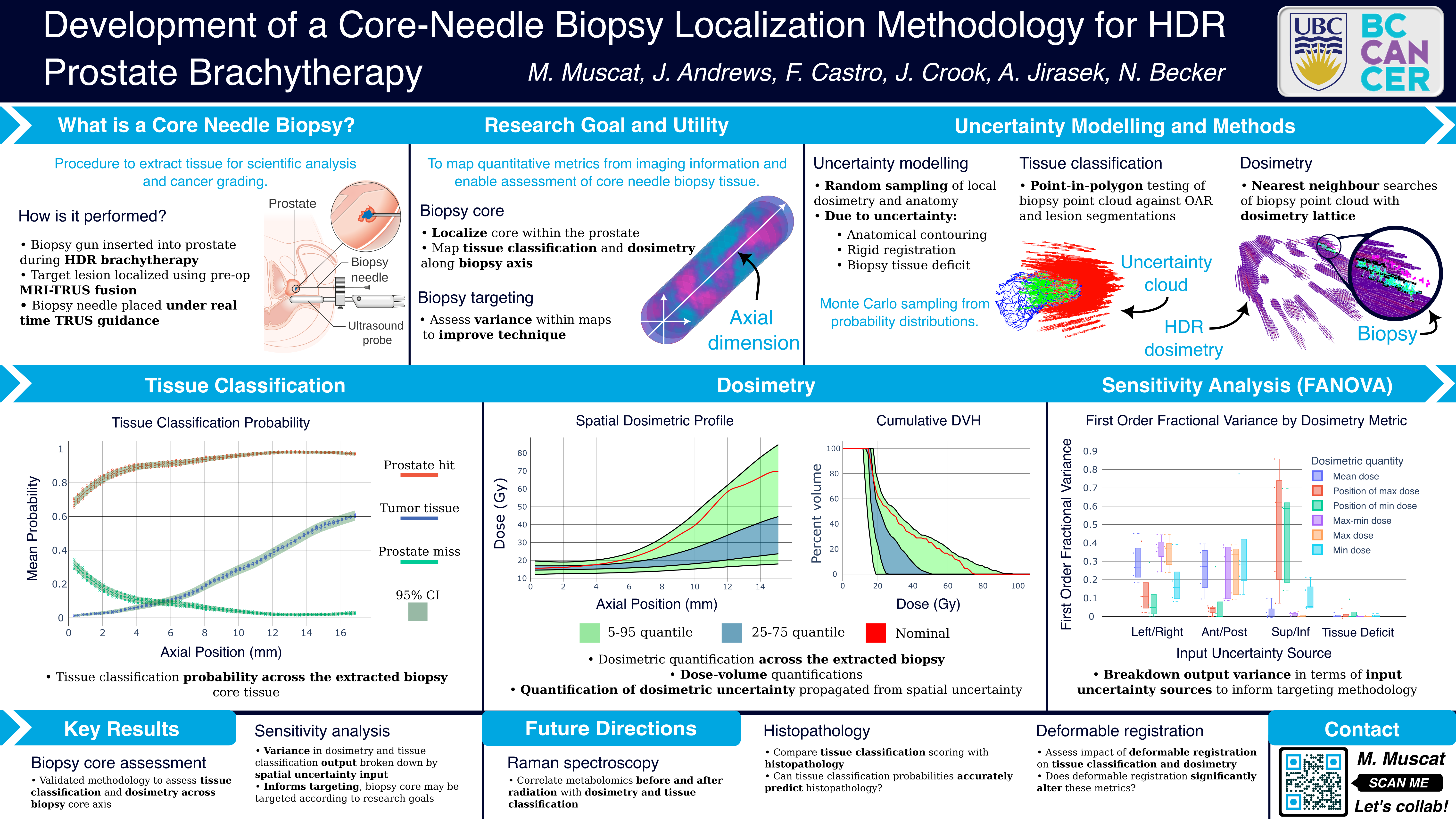
Abstract
Purpose: Clinicians who perform prostate high dose-rate (HDR) brachytherapy are increasingly utilizing multiparametric magnetic resonance (MR) to identify and target dominant intraprostatic lesions (DILs). Prostate HDR also offers a unique opportunity for the acquisition of tissue samples via core-needle biopsy, and Raman spectroscopy may provide an observational window for which to monitor radiation treatment. In this work, we localize the core-needle biopsy within the patient to assess tissue type (DIL versus normal prostate), and radiation dose variation across the core. We further assess the impacts of the biopsy localization uncertainty on dose and tissue classification variation.
Materials and Methods: Ultrasound imaging was acquired during the core needle biopsy procedure, and the biopsy position identified through ultrasound and MR fusion. To estimate the dose and tissue classification across the biopsy core, and to assess the localization uncertainty, two methods were employed to simulate both systematic and random uncertainty. The first is a Monte-Carlo based method, where uncertainties are randomly sampled. The second is through the use of a non-parametric constant Gaussian kernel regression (convolution) of the background dosimetric and tissue classification lattice. The degree of uncertainty in these methods are controlled by the sampling distribution
parameters and kernel parameters respectively. The magnitude of localization uncertainty was varied in both methods to determine the most significant directions of uncertainty, as well as to determine upper bounds on the localization uncertainty vectors to achieve discernible variation across the biopsy tissue core.
Results: Preliminary analysis was performed using constant 1, 3, and 5 mm uncertainty (σ1, σ3, σ5) in each of the orthogonal directions of the patient coordinate system for all structures. The analysis was performed on biopsy core tissue samples of lengths (15.2 ± 0.1) mm (A) and (17.0 ± 0.1) mm (B). The biopsy tissue cores were voxelized in 1 mm × 0.6 mm (length and radius) cylindrical volumes, with approximately 10000 samples per voxel. This provided dosimetry profiles ranging from (σ1: (16 ± 1) Gy, σ5: (15 ± 4) Gy) (A) and (σ1: (22 ± 2) Gy, σ5: (22 ± 14) Gy) (B) at the inferior voxel, to (σ1: (57 ± 29) Gy, σ5: (23 ± 14) Gy) (A) and (σ1: (34 ± 12) Gy, σ5: (28 ± 16) Gy) (B) at the superior voxel after the first treatment fraction. The probability of the core tissue classification varied from σ1 : (100 ± 0)% (A and B) prostate and (0 ± 0)% (A and B) DIL at the inferior voxel; to σ1 : (95 ± 2)% (A) and σ1 : (98 ± 1)% (B) DIL at the superior voxel.
Conclusions: Preliminary results suggest that localization uncertainties on the order of a millimeter allow for statistically discernible variation in both the dosimetry and tissue classification within the biopsy core tissue sample. Future work will look to assess and identify specific sources of uncertainty such as deformable registration, and provide patient specific estimates from our outcome assessment model