Abstract
Background: Intrauterine devices (IUD) are a reliable and reversible form of long-term contraception. However, procedural pain with insertion is a concern and deterrent for many patients, especially those who are nulliparous. Although IUD insertion is known to cause discomfort, there are not set guidelines regarding procedural analgesia and the devices are frequently inserted without any local pain control. Reports suggest that providers believe the pain associated with injecting a local analgesic into the cervix is not worth the potential benefit it provides during the procedure. Therefore, the efficacy of topical lidocaine in procedural pain control warrants investigation.
Objective: The objective of this systematic review is to investigate the efficacy of topical lidocaine used to reduce pain during IUD insertion.
Methods: PubMed was searched for articles including the words [lidocaine] and [intrauterine device] with [spray], [gel], [cream], or [topical]. This review includes randomized control trials published within the last 10 years, up to October 2022. Only trials that evaluated topical forms of lidocaine that are available on market were included. Trials were required to have a group receiving topical lidocaine as the only additional intervention compared to the control group. Trials without full text available were not included in the review.
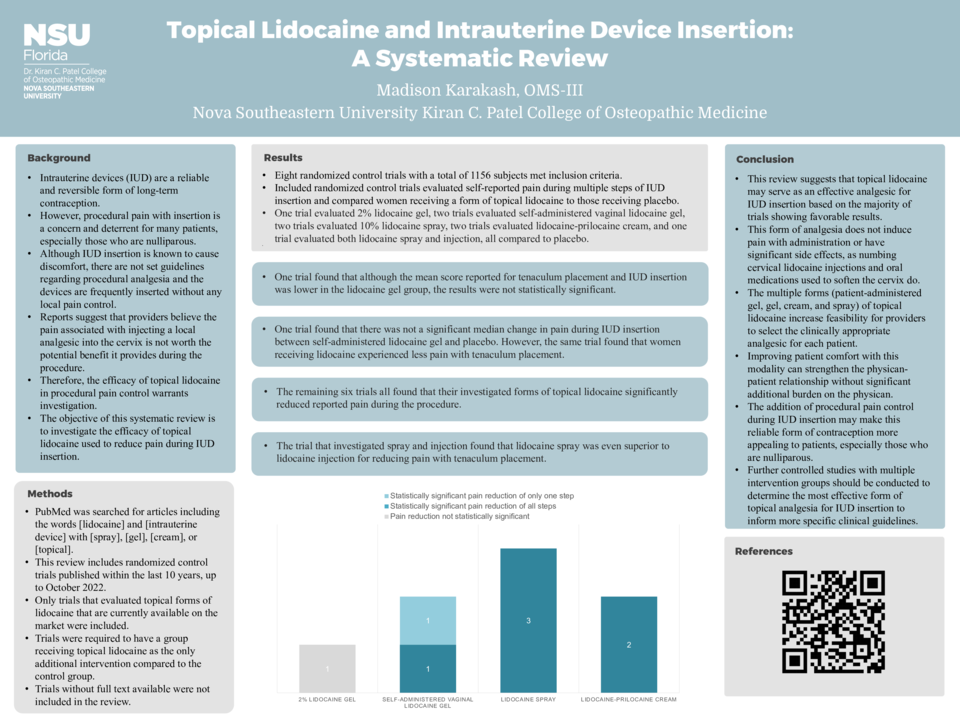
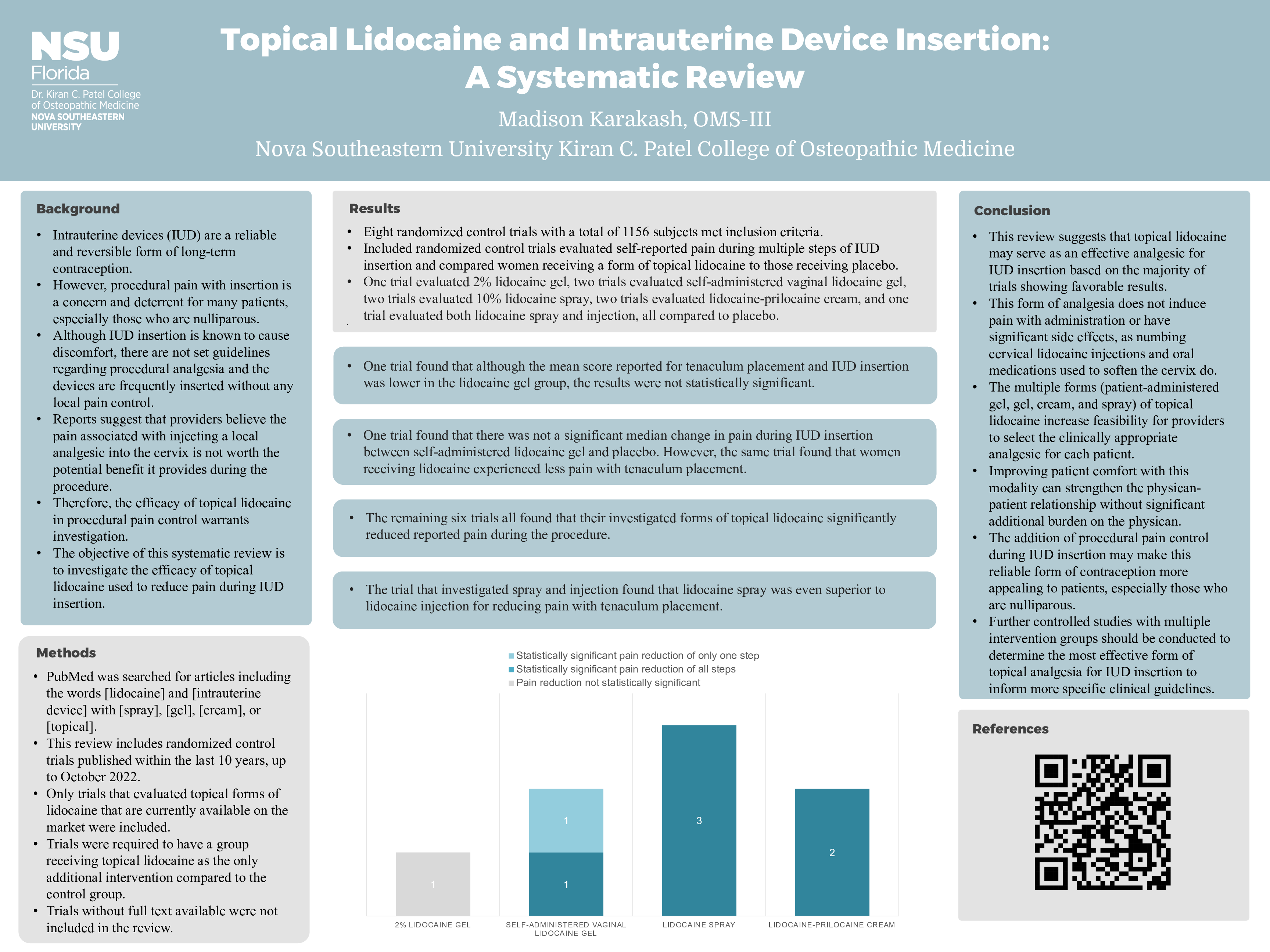
Results: Eight randomized control trials with a total of 1156 subjects met inclusion criteria. Included randomized control trials evaluated self-reported pain during multiple steps of IUD insertion and compared women receiving a form of topical lidocaine to those receiving placebo. One trial evaluated 2% lidocaine gel, two trials evaluated self-administered vaginal lidocaine gel, two trials evaluated 10% lidocaine spray, two trials evaluated lidocaine-prilocaine cream, and one trial evaluated both lidocaine spray and injection, all compared to placebo. One trial found that although the mean score reported for tenaculum placement and IUD insertion was lower in the lidocaine gel group, the results were not statistically significant. One trial found that there was not a significant median change in pain during IUD insertion between self-administered lidocaine gel and placebo. However, the same trial found that women receiving lidocaine experienced less pain with tenaculum placement. The remaining six trials all found that their investigated forms of topical lidocaine significantly reduced reported pain during the procedure. The trial that investigated spray and injection found that lidocaine spray was even superior to lidocaine injection for reducing pain with tenaculum placement.
Conclusion: This review suggests topical lidocaine may serve as an effective analgesic for IUD insertion based on the majority of trials showing favorable results. This form of analgesia does not induce pain with administration or have significant side effects. The multiple forms (patient-administered gel, gel, cream, and spray) of topical lidocaine increase feasibility for providers to select the clinically appropriate analgesic for each patient. Further controlled studies with multiple intervention groups should be conducted to determine the most effective form of topical analgesia for IUD insertion to inform more specific clinical guidelines.