Abstract
Objectives: Glioblastoma relapse is a current oncology challenge. Radiosurgery (SRS) may be an effective treatment option. A large number of studies described the value of SRS, but this technique still remains with low indication. This retrospective study was carried out in order to evaluate our experience in patients treated for glioblastoma relapse.
Methods: Between January 2000 and March 2016, 27 patients with glioblastoma multiforme, grade IV glioma, relapse were treated with 40 SRS, they a mean age of 57.6 years (range 24-79). These patients had previously been treated with surgery, chemotherapy (CTX) and radiotherapy (EBRT) according to the Stupp protocol. The number of lesions treated with SRS is 1 to 4 (median 1,44) and the mediam volume of lesions is 16.23 cc (0.636-58.48). The prescribed dose was from 10 Gy to 18 Gy (median-14.33 Gy) 1 one fraction. Follow-up duration is 16.82 months (4-48). Simultaneously with SRS all patients with relapse received CTX with bevacizumab (16 patients) or temozolomide (11 patients).
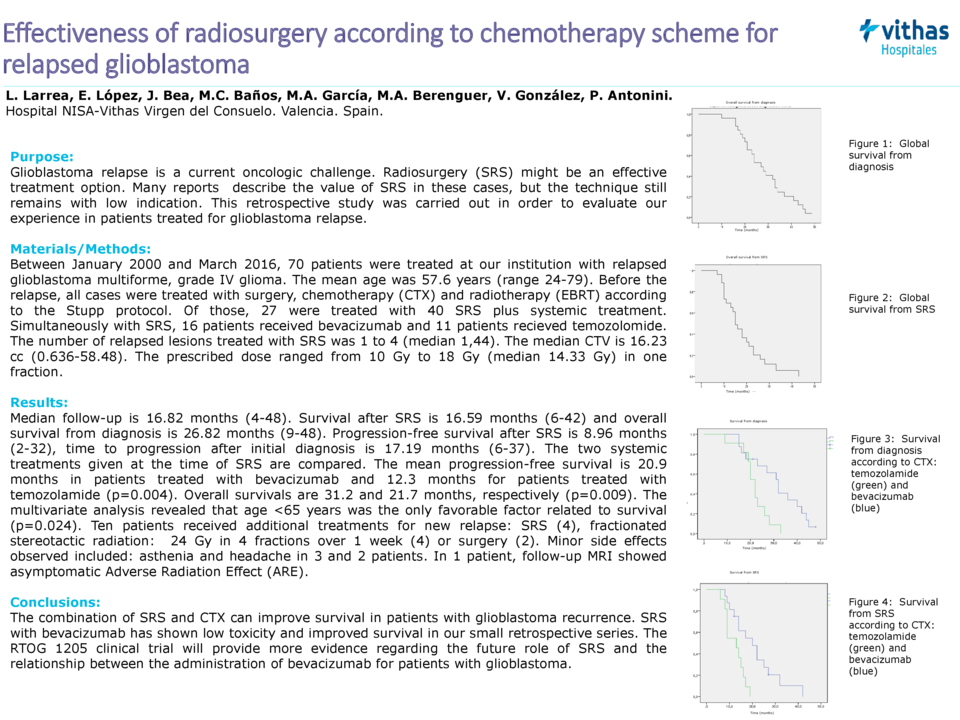
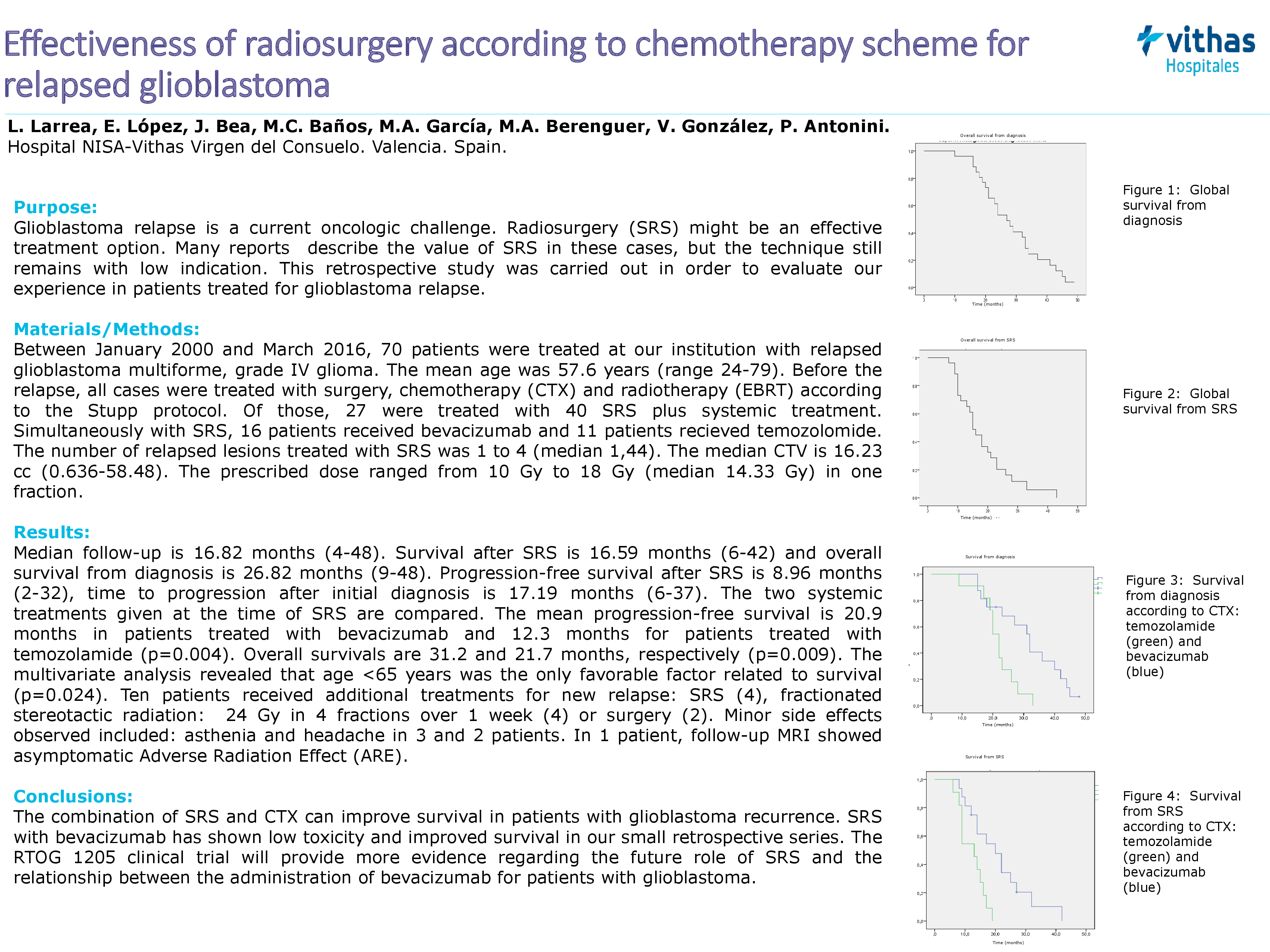
Results: Survival time after SRS is 16.59 months (6-42) and overall survival is 26.82 months (9-48) from diagnosis. Progression-free survival after SRS is 8.96 months (2-32), time to progression after initial diagnosis is 17.19 months (6-37). Two CTX regimen at the time of SRS are comparated. The mean progression-free survival in patients treated with bevacizumab is 20.98 months, temozolomide is 12.27 (p=0.004). Overall survival is 31.27 months and 21.73 months, respectively (p=0.009). The multivariate analysis revealed that age <65 years was the only favorable factor in the treatment (p=0.024). Ten patients received additional treatments for new relapse SRS (4), FSRT (4) or surgery (2). Minor side effects were asthenia and headache in 3 and 2 patients. In 1 patient MR was observed Adverse Radiations Effect (ARE).
Conclusions: The combination of SRS and CTX treatment in combination results can improve survival of patients with tumor recurrence of glioblastoma. SRS with bevacizumab has been with low toxicity and with better survival in our small number of patients. The RTOG 1205 clinical trial will provide a greater level of evidence regarding the future role of SRS and the relationship between the administration of Bevacizumab for patients with glioblastoma.